Are We Providing Enough Information to the Pathologists? An Audit of Filling of Histopathology Request Forms for Surgically Resected Tumours
By Mishaal Shan Siddiqui1, Suresh Kumar2, Dinesh Kumar3, Saad Khalid1, Wardah Hassan1, Mahima Khatri1Affiliations
doi: 10.29271/jcpsp.2024.04.484ABSTRACT
Objective: To analyse quantitatively the adequacy of demographics of clinical information and highlight specific areas of neglect, by assessing the adequacy of filling out histopathology request forms.
Study Design: Clinical Audit.
Place and Duration of the Study: Department of Pathology, Dow University of Health Sciences (DUHS), Karachi, Pakistan, from January to September 2021.
Methodology: A retrospective audit was carried out on the request forms of surgically resected tumours and biopsies. The recorded details of the patients’ demographics and biopsy, clinical history and examination, and intraoperative findings were analysed.
Results: Out of 175 forms, patients' names were written in 174 (99.4%) while medical record numbers were written in 113 (64.6%). The doctors’ names were given in 172 (98.3%) forms and phone numbers in 34 (19.4%). A clinical diagnosis was provided in 164 (93.7%) forms, while 152 (86.9%) forms correctly entered the biopsy site. Sixty-seven (38.3%) forms included the correct nature of the biopsy. Relevant operative details were provided in half of the forms. Symptoms and their duration were mentioned in 144 (82.3%) and 100 (57.1%), respectively. The form-filling rate was the same for both benign and malignant tumours.
Conclusion: This study shows that in a significant proportion of cases, complete relevant information is not provided to the histopathologists on request forms for logistics.
Key Words: Histopathology, Request forms, Tumours, Audit.
INTRODUCTION
Pathological examination of surgically removed tissues under a microscope remains the gold standard for the diagnosis of a variety of illnesses, such as malignancies, autoimmune disorders, infections, and other conditions where laboratory or radiographic studies are insufficient to reach a conclusion. However, when considered apart from the clinical context, histological results can be perplexing and misleading.1 Therefore, it is imperative that the physicians ordering histology provide the histopathologists with pertinent clinical history, radiological details, and other crucial information. These help in the diagnosis and assessment of illness progression or prognosis.2
Unfortunately, there is a relative lack of adherence to such standard criteria, and pathologists from all over the globe have expressed similar worries.3,4 Failure to give crucial information might lead to major diagnostic mistakes and needless reporting, and consequently, management delays.5 This is crucial when dealing with potentially aggressive tumours since even small delays might cause the illness to advance and necessitate changing the treatment plan. Such issues often arise in settings where quality control standards are not rigorously followed.3,4 The inability to complete the histopathological request forms might be due to a variety of circumstances. These include failing to recognise the significance of this information in diagnosis, the need to expedite things, and getting inadequate training on how to fill out the required parts.6
The aim of this internal audit was to look at the adequacy of filling out forms received by the pathology department for the pathologic study of surgically removed specimens.
METHODOLOGY
This retrospective audit was conducted in the Department of Pathology, Dow University of Health Sciences, Karachi, Pakistan. The department of pathology mainly receives histopathology requests from the affiliated 1,900-bed tertiary care teaching hospital. Histopathology request forms received by the department from January to September 2021 were assessed for completeness, accuracy, and consistency.
All histopathology request forms were included that were filled out by the hospital’s surgical and gynaecological departments for any benign or malignant tumour or ulcerated lesion. Clinically obvious non-tumour inflammatory conditions, such as appendicitis, cholecystitis, viscus perforation secondary to trauma, and other pathologies, such as pelvic organ prolapse were excluded.
In a predesigned proforma, relevant data were extracted from the histopathology request forms. Relevant data elements included the name of the patient, age, gender, medical record (MR) number, ward, date of biopsy, date of sending the specimen, nature of the biopsy, site of biopsy, relevant history of the patient, clinical diagnosis, operative findings, rough illustration of the site of biopsy, and specimen tagging information. The request forms were matched with the respective histopathology reports via MR numbers. The use of formalin as a preservative and other important diagnostic information were obtained from histopathology reports to assess the impact of adequate filling of request forms on a patient's diagnosis.
Sections of clinical history, examination, and operative findings were broken down into several components to help determine their adequacy and accuracy. The sections of clinical history and examination findings were categorised into details regarding patients' symptoms, their duration, and any risk factors or red flags in addition to the local lesion's examination, including its site, size, extent, and relationship with nearby structures. Operative details included information about the size, site, and gross appearance of the lesion, along with the intraoperative status of adjacent structures. If there was a disagreement in the assessment concerning whether the correct nature and site of the biopsy were specified in the request form, an experienced pathologist (SK) was consulted to reach a consensus.
Data were entered and analysed using Statistical Package for the Social Sciences software (SPSS version 25.0; IBM Corporation, Armonk, NY, US). Quantitative variables were presented as mean ± standard deviation (SD). Frequencies and percentages were reported for categorical variables and to define the filling rate of the request form sections. The chi-square test was used for comparison between different categorical variables. If Chi-square assumptions were not met, the Fisher exact test was used. A p-value of ≤ 0.05 was considered significant for all the results.
RESULTS
During the study period, 175 forms were assessed, consisting of 132 females and 32 males, while 11 (63%) forms had no details regarding the gender of the patients. The mean age of patients was 35.23 ± 21.27 years and the mean time to diagnosis was 12 ± 6 days.
Patients' names were written in 174 (99.4%) forms, while MR and bed numbers were reported in 113 (64.6%) and 92 (52.8%) forms, respectively. The doctors’ names were given in 172 (98.3%) and the doctors’ phone numbers in 34 (19.4%) forms. The patients’ phone numbers were mentioned in 49 (28%) forms. Most of the forms were received from the departments of gynaecology and surgery, while 4 (2.3%) of forms had no ward specifications. Table I shows the baseline characteristics of the request forms.
Table I: Descriptive characteristics of included histopathology request forms (n=175).
|
Characteristics |
Mean |
SD |
|
Age |
35.23 |
21.27 |
|
|
Number (n) |
Percentage (%) |
|
Gender |
|
|
|
Male |
32 |
18.3 |
|
Female |
132 |
75.4 |
|
Missing |
11 |
6.3 |
|
Wards |
|
|
|
Incompletely filled / Missing |
4 |
2.3 |
|
Nature of biopsy |
|
|
|
Incompletely filled / Missing |
44 |
25.1 |
|
Site of biopsy |
|
|
|
Breast |
29 |
16.6 |
|
Ovary |
18 |
10.3 |
|
Skin / subcutaneous tissue |
18 |
10.3 |
|
Uterus with ovaries and tubes |
16 |
9.1 |
|
Eye |
12 |
6.9 |
|
Cervix |
11 |
6.3 |
|
Ileum / cecum / colon / rectum |
10 |
5.7 |
|
Endometrium |
8 |
4.6 |
|
Uterus |
6 |
3.4 |
|
Esophagus / antrum / duodenum |
6 |
3.4 |
|
Mucosa |
5 |
2.9 |
|
Axilla/axillary lymph nodes |
4 |
2.3 |
|
Others |
10 |
5.7 |
|
Incompletely filled / missing |
22 |
12.6 |
|
Clinical Diagnosis |
|
|
|
Ovarian mass / cyst / adnexal mass |
25 |
14.3 |
|
Breast lump / fibroadenoma |
20 |
11.4 |
|
GI tumours / masses |
15 |
8.6 |
|
RPOC / Molar pregnancy |
13 |
7.4 |
|
Skin Pathologies (BCC / Melanoma / Nevus / Boil) |
11 |
6.3 |
|
Fibroid |
10 |
5.7 |
|
Dermoid cyst |
9 |
5.1 |
|
Endometrial polyp / cyst / hyperplasia / carcinoma |
8 |
4.6 |
|
Cervical mass / carcinoma |
8 |
4.6 |
|
Sebaceous cyst |
7 |
4.0 |
|
Breast carcinoma |
7 |
4.0 |
|
Eye pathologies |
6 |
3.4 |
|
Lipoma / fibroma |
5 |
2.9 |
|
Others |
20 |
11.4 |
|
Incompletely filled / undiagnosed |
11 |
6.3 |
|
Nature of lesion |
|
|
|
Benign |
122 |
69.7 |
|
Pre-malignant / Malignant |
50 |
28.6 |
|
Missing |
3 |
1.7 |
|
TAHBSO: Total abdominal hysterectomy with bilateral salpingo-oophorectomy; RPOC: Retained products of conception; BCC: Basal cell carcinoma. |
||
A clinical diagnosis was provided in 164 (93.7%) forms. Additionally, 152 (86.9%) request forms correctly entered the biopsy site, whereas only 67 (38.3%) forms reported the correct nature of the biopsy. Histology identified 122 (69.7%) cases as benign, while 50 (28.6%) were malignant or premalignant. Figures 1 and 2 demonstrate the frequencies of reporting the key clinical history and examination findings along with operative details. Data regarding radiology was given in 16 (9.1%) samples, and prior histology reports were provided in 33 (18.9%) cases. One hundred and sixty-five (94.3%) of the biopsied samples were preserved in formalin.
Overall, 14 (8%) reports were inconclusive or yielded nonspecific results and 11(78%) of them had incompletely filled forms. Meanwhile, the remaining 161 (92%) reports provided a conclusive diagnosis. Out of these, 75 (46.58%) had relatively complete forms and the remaining 86 (53%) had incomplete forms.
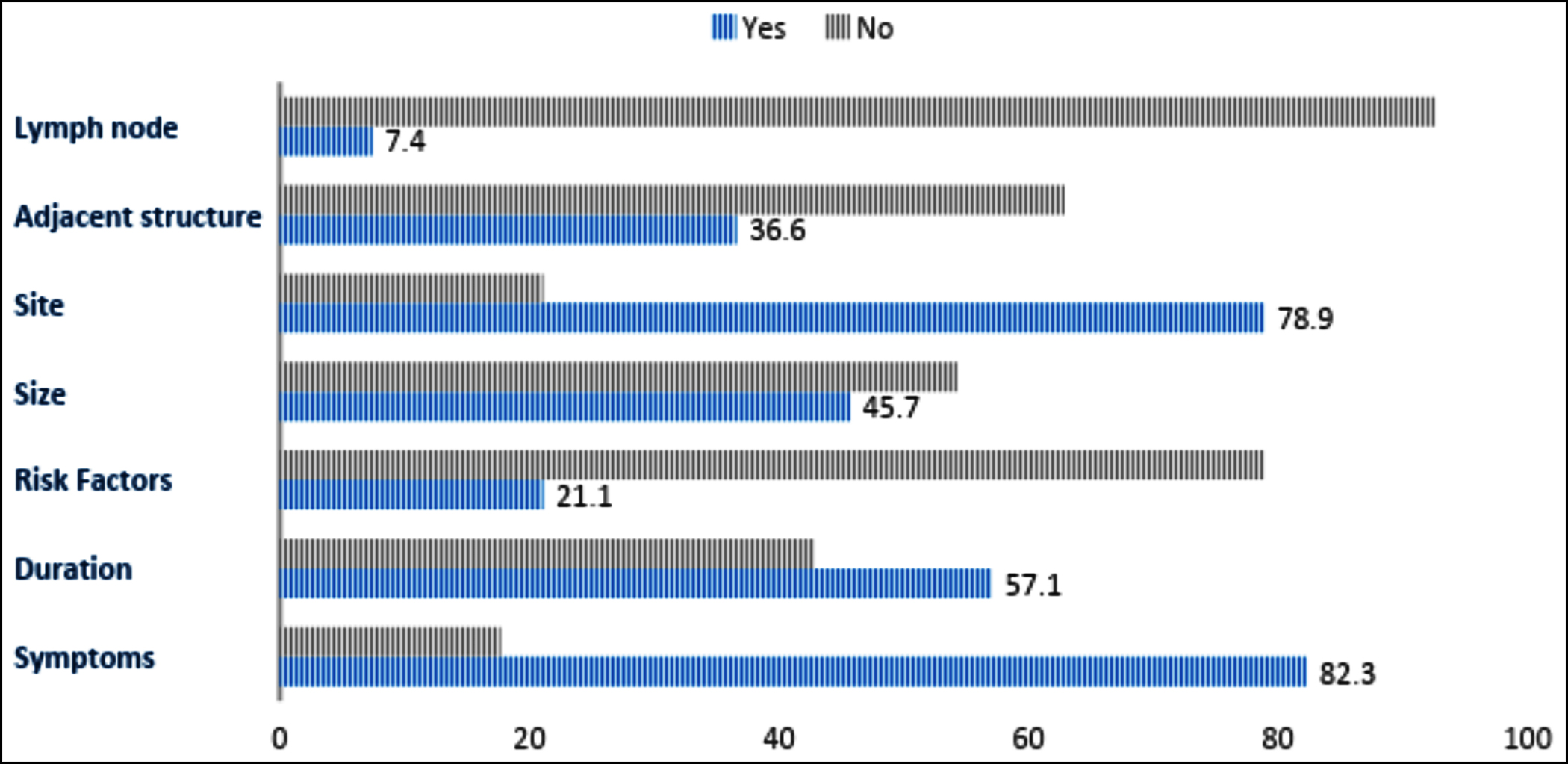 Figure 1: Adequacy of filling of clinical history and examination.
Figure 1: Adequacy of filling of clinical history and examination.
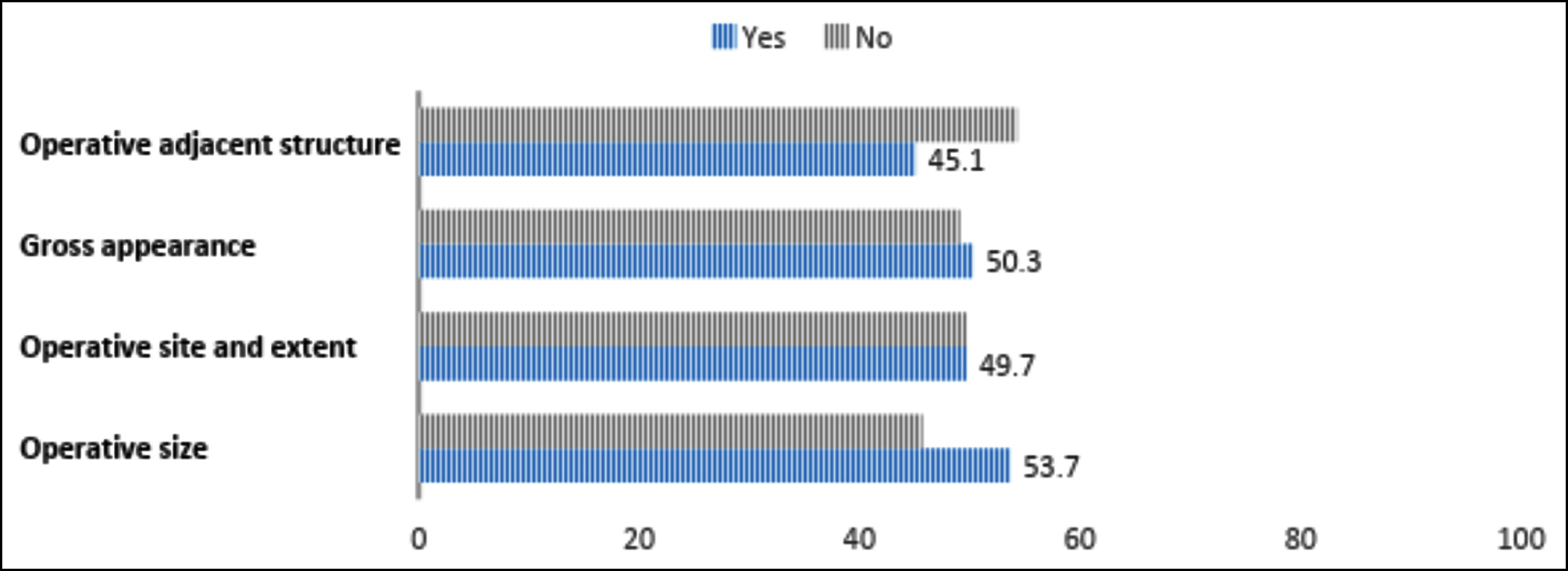 Figure 2: Adequacy of provision of operative findings.
Figure 2: Adequacy of provision of operative findings.
A significant association was observed between the reporting of operative size and the type of ward with gynaecology wards having a better reporting frequency (p < 0.001). However, no significant relationship was found between the type of ward and biopsy site (p = 0.18), the reporting of symptoms (p = 0.15), and their duration (p = 0.16). No association was found between the ward type and the correctness of the nature of the biopsy (p = 0.472) or site of biopsy (p = 0.13).
No significant association was found between the last menstrual period (LMP) and the site of biopsy (p = 0.42) or clinical diagnosis (p = 0.53). Similarly, no significant association was drawn between benign vs. malignant diagnosis and the type of ward (p = 0.46), reporting of symptoms (p = 0.14), duration of symptoms (p = 0.52), radiology (p = 0.07), the gross appearance of the lesion (p = 0.38) or status of adjacent structures (p=0.145). Mean time to diagnosis for benign and malignant conditions was also insignificant at 12.8± 6.9 days and 11.12±4.48 days, respectively (p = 0.1).
Rectification:
Following the completion of the initial audit, the results revealed several areas of concern regarding the completeness and accuracy of histopathology request forms. In response to these findings, the healthcare institution implemented a series of correction measures aimed at improving the information provided to the pathologists. The objective was to enhance diagnostic accuracy, reporting efficiency, and overall patient care by ensuring that the necessary clinical information was adequately recorded on the request forms.
Physician Education and Training: A comprehensive educational program was developed to raise awareness among clinicians about the significance of providing complete and relevant clinical information on histopathology request forms. Workshops and seminars were conducted to educate physicians on the impact of inadequate information on diagnostic accuracy and patient outcomes. Physicians were guided on how to fill out the forms correctly, emphasising the importance of specific fields, such as patient demographics, clinical history, operative details, and the correct nature and site of the biopsy.
Standardised and Streamlined Request Forms: The histopathology request forms were redesigned to incorporate clear and concise options for various fields. By providing pre-defined choices, clinicians were guided to select appropriate responses, reducing the likelihood of leaving sections blank. Mandatory fields were implemented for essential information, such as patient name, age, clinical diagnosis, biopsy site, and doctors’ contact details. This standardisation streamlined the process of form-filling and minimised the chances of missing data.
Digitisation of Record-Keeping: To enhance the accuracy and accessibility of data, the institution transitioned from manual record-keeping to a computerised system for histopathology requests. Physicians were encouraged to use the electronic system, which reduced the risk of missing information and improved the overall efficiency of the process. The computerised system also generated alerts and reminders to ensure that mandatory fields were completed before the form could be submitted.
Quality Control Measures: Regular quality control checks were instituted to review a sample of filled-out request forms periodically. This process provided valuable feedback to clinicians and identified any persisting issues. Constructive feedback encouraged physicians to be more diligent in filling out the forms, contributing to continuous improvement.
Re-evaluation and improvement:
After the implementation of the rectification measures, a follow-up audit was conducted to assess the effectiveness of the interventions. The objective was to determine whether the steps taken to improve the filling of histopathology request forms resulted in positive changes and adequacy of information provided to the pathologists.
Data Collection and Comparison: Similar to the initial audit, histopathology request forms for surgically resected tumours were collected over a specific period for the re-evaluation. The data collected in this phase were compared with the findings from the initial audit to identify any improvements.
Assessment of Compliance: The re-evaluation assessed the degree of compliance with the rectification measures. It determined how many forms now contained complete and relevant information compared to the initial audit. The rate of adherence to mandatory fields and the utilisation of optional fields were also evaluated.
Diagnostic Outcomes: The correlation between the quality of the information provided and the histopathology reports was analysed to evaluate the impact on diagnostic accuracy. The assessment aimed to identify whether improved information led to more accurate and reliable diagnoses.
Feedback and Further Improvement: Based on the findings of the re-evaluation, further feedback and training were provided to clinicians if necessary. Continuous improvement strategies were implemented to sustain the positive changes and address any remaining issues.
Documentation of Results: The results of the re-evaluation were documented and shared with relevant stakeholders, including the clinical and pathology departments. The findings highlighted the success of the interventions and served as a basis for future quality improvement initiatives.
The re-evaluation demonstrated significant improvements in the completeness and accuracy of histopathology request forms for surgically resected tumours. Physicians showed increased compliance with the mandatory fields, and the rate of incomplete forms decreased substantially. The implementation of standardised forms and the digitisation of record-keeping streamlined the process and reduced the chances of missing information. Moreover, the correlation between improved information and diagnostic accuracy indicated positive outcomes in patient care. The successful outcome of the audit and subsequent improvements emphasised the importance of regular audits in ensuring the provision of sufficient information to pathologists for enhanced patient outcomes.
DISCUSSION
The results from this study show substandard provision of clinical data to the histopathologists. While many countries follow a centralised, computerised system to fill in and retrieve data across multiple devices. In third-world countries like Pakistan, where manual data entry prevails, continue to suffer from such commotions.
Name, age, and gender not only provide important identification information but are important to record correctly and avoid any errors in reporting, mixing of forms, or even maintaining a proper database for later use. In the present study, patients' names and ages were the most frequently filled elements on request forms. A study from Ghana revealed patients' age and gender to be missing from 25.6% and 32.7% of forms, respectively.7 The phone numbers of the patients were missing in around three-quarters of the forms, and the doctors’ phone numbers were absent in 80.57% of the cases. This not only leads to a delay in communicating urgent, important results but also creates a hindrance in obtaining relevant information from the doctor or patient, which might prove useful in reporting, especially in assessing the prognosis in malignant cases.3 Relevant clinical history and preoperative data provide a number of crucial supporting roles in histological diagnosis. Another study from this country found that 34% of forms did not contain relevant clinical histories.8 For surgical specimens, the operation site, its appearance in the body, and the condition of neighbouring structures, such as scarring, disease extension, or any other abnormalities, can all influence the histopathologist's approach.9,10 In the present research, around half of the forms provided details about the size, location, appearance of the lesion, and condition of the neighbouring organs. Clinical histories revealed symptoms and the duration of such symptoms in 82.3% and 57.1% of patients, respectively. Other studies also showed low filling rates of clinical history at 14% and 35% respectively.11,12
Little attention is paid to the LMP in gynaecologic specimens. Only 25 of the 128 female participants in this study had LMP recorded. This holds particular importance for endometrial and ovarian samples that may be interpreted differently in different phases of the menstrual cycle.13-15
The pathologist can better understand the clinical picture if clinicians provide a crude representation of the tumour or lesion on the body. It could stop any small or difficult-to-see lesion from being lost to the surrounding tissue. However, only 6.3% of the forms in the present study provided this information.
A software that can either rapidly extract the crucial data or provide the doctor and histopathologist with an easy way to upload and see it without having to complete the paper-based forms may be the key to finding a more long-lasting solution. However, because of the nature of manual data entry, this phase seems to be fairly challenging to complete in the majority of wards. A simpler approach to improving reporting standards at this time is to provide attending physicians with clear guidance on how to fill out the forms correctly. It could be more efficient to change request forms to limit user input, for example. It may be done by listing optional headers alongside those that are necessary. It is equally important to guide and train the requesting physicians about the importance and correct manner of filling out these forms through continued medical education seminars and conference meetings. This point should also be repeatedly highlighted in institutional multidisciplinary tumour board meetings. Finally, forms should be designed in such a way that they allow clinicians to choose or tick off most of the options rather than spending time writing or typing the details.
CONCLUSION
The filling of histopathology request forms remains suboptimal in this setup. Consistent strategies must be adopted to ensure their completeness. These include digitising the record-keeping process, in which filling out the relevant sections is made mandatory for online submission of forms. It is also necessary to sensitize the doctors about the importance of providing relevant clinical information.
COMPETING INTEREST:The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
MSS: Conceptualisation and writing the original draft.
SK: Final approval of the manuscript.
DK: Writing draft and data collection.
FS, SHA: Data collection.
SK: Statistical Analysis.
WH: Writing review.
MK: Editing of the manuscript.
All authors approved the final version of the manuscript for publication.
REFERENCES
- Abbasi F, Asghari Y, Niazkhani Z. Information adequacy in histopathology request forms: A milestone in making a communication bridge between confusion and clarity in medical diagnosis. Turk Patoloji Derg 2023; 39(3):185-91. doi: 10.5146/tjpath.2022.01595.
- Laboratory request forms [Internet]. 2023. Available from: https://archive.uhb.nhs.uk/laboratory-request-forms.htm.
- Nutt L, Zemlin AE, Erasmus RT. Incomplete laboratory request forms: The extent and impact on critical results at a tertiary hospital in South Africa. Ann Clin Biochem 2008; 45(Pt 5):463-6. doi: 10.1258/acb.2008.007252.
- Oyedeji OA, Ogbenna AA, Iwuala SO. An audit of request forms submitted in a multidisciplinary diagnostic center in Lagos. Pan Afr Med J 2015; 20:423. doi: 10.11604/pamj. 2015.20.423.5778.
- Wonodi W, Jaja I, Ogolodom M, Mbaba A, Alazigha N. Evaluation of reasons for non-complete filling of investi-gation request forms by medical doctors in rivers state, nigeria. Crit Care Obst Gyne 2021; 7(3):31.
- Adegoke O, Idowu A, Jeje OA. Incomplete laboratory request forms as a contributory factor to preanalytical errors in a Nigerian teaching hospital. Afr J Biochem Res 2011; 5(3): 82-5.
- Olayemi E, Asiamah-Broni R. Evaluation of request forms submitted to the haematology laboratory in a Ghanaian tertiary hospital. Pan Afr Med J 2011; 8:33. doi: 10.4314/ pamj.v8i1.71148.
- Sharif MA, Mushtaq S, Mamoon N, Jamal S, Luqman MJPJoMS. Clinician's responsibility in pre-analytical quality assurance of histopathology. Pak J Med Sci 2007; 23(5): 720.
- Raab SS, Oweity T, Hughes JH, Salomao DR, Kelley CM, Flynn CM, et al. Effect of clinical history on diagnostic accuracy in the cytologic interpretation of bronchial brush specimens. Am J Clinical Pathol 2000; 114(1):78-83. doi:10.1309/4099-qald-nvgf-tm4g.
- Hoda SA. Underwood’s pathology: A clinical approach. Am J Clinical Pathol 2019; 151(1):127. doi:10.1093/ajcp/aqy140.
- Priyadharisini, Porko S, Barman PP. Audit of histopathology request forms submitted in laboratory of a tertiary care hospital. J Med Sci Clin Res 2019; 07(04):1085-89. doi:10. 18535/jmscr/v7i4.178.
- Kipkulei JC, Lotodo TC. Evaluation of the completeness in the filling of laboratory request forms submitted to the haematology laboratory at a tertiary hospital in Kenya. Health 2019; 11(7):862-8. doi: 10.4236/health. 2019.11 7069.
- Agency A| W Dallas Integrated Creative Communications. SASGOG Pearls of Exxcellence | The Society for Academic Specialists in General Obstetrics & Gynecology. [cited 2023 Aug 1]. Interpretation of benign endometrial biopsy report when evaluating abnormal uterine bleeding. Available from: https://www.exxcellence.org:443/list-of-pearls/interpretation-of-benign-endometrial-biopsy-report-when-evaluating-abnormal-uterine-bleeding.
- Atanda AT, Raphael S. Role of surgeons in determining outcome of histopathology specimens. Niger J Surg 2013; 19(2):68-72. doi: 10.4103/1117-6806.119242.
- Moifo B, Kamgnie MN, Fointama NF, Tambe J, Tebere H, Fotsin JG. Évaluation de la conformité des demandes d'examens d'imagerie médicale: Une expérience en Afrique subsaharienne [Assessment of the completeness of medical imaging request forms in a sub-Saharan African setting]. Med Sante Trop 2014; 24(4):392-6. French. doi: 10.1684/ mst.2014.0382.